Abstract
INTRODUCTION:
Hispanic patients with acute lymphoblastic leukemia (ALL) are historically known to have poor outcomes compared to non-Hispanic patients which may be associated with healthcare disparities, although further elucidation is needed.
Since we take care of a large population of Hispanic patients, we now report further updated outcomes in Hispanic compared to Non-Hispanic ALL patients using the modified USC ALL regimen in the era of novel agents like blinatumomab and inotuzumab.
METHODS:
This retrospective chart review included adults 18 years old with newly diagnosed Ph-negative ALL (2016-2020). Primary objectives were 3-year over-all survival (OS), event-free survival (EFS) and disease-free survival (DFS) stratified by MRD flow status and blinatumomab use. Secondary objectives were complete remission/complete remission with incomplete recovery (CR/CRi), MRD by flow, descriptive statistics of patients stratified into Hispanic and non-Hispanic cohorts evaluated using Fisher's exact test. OS, DFS, EFS reported through Kaplan Meier curves and Log-rank tests. Two-sided p-value ≤0.05 was significant.
RESULTS:
121 Ph-negative ALL patients were reviewed. 87 Hispanic patients (HP) and 34 non-Hispanic patients (NHP). Median ages in HP and NHP were 39 and 35 years; median BMI 29 and 26.9 kg/ m2 (p=0.42-0.51), respectively. About equal males and females in HP while NHP had 70.6% males compared to 29.4% females (p=0.08). Both HP and NHP were mainly Ph-negative ALL, 50.6% vs 47.1%, 25.4% vs 20.6% were Ph-like, respectively (p=0.884). Most of our population had unfavorable risk by NCCN, 86.8% in HP and 90.5% in NHP (p=0.99). 71.1% HP and 62.5% NHP received PEG during induction 1 (p=0.37), and 69.6% of HP and 63% of NHP received PEG during induction 2 (p=0.52).
After induction 1: 91.1% of HP and 80% in NHP achieved CR/Cri (p=0.18). 55.7% of HP and 40.9% of NHP achieved MRD negativity by flow (p=0.23). After induction 2: 92.7% of HP and 79% of NHP achieved CR/CRi, with 7.3% in HP being refractory compared to 21.1% of NHP (p=0.19). 73.3% of HP and 64.7% of NHP achieved MRD negativity by flow (p=0.54). 26.4% of HP and 35.3% of NHP had relapse (p=0.33), 4.6% of HP and 20.6% of NHP had refractory disease (p=0.01), while 4.6% of HP compared to 20.6% of NHP died (p=0.27).
Blinatumomab was given in 35.6% of HP and 32.3% of NHP (p=0.73). Meanwhile only 6.9% of HP and 2.9% of NHP received inotuzumab (p=0.67).
39.1% of HP underwent allogenic hematopoietic stem cell transplant (allo-HSCT) versus 26.5% in NHP (p=0.19). 26.4% HP and NHP who received blinatumomab did not undergo allo-HSCT. 3.4% of HP and 11.8% of NHP underwent CAR-T (p=0.096).
3y OS was 82.4% in HP vs 75.8% in NHP (p=0.18). 3y EFS was 58.6% in HP vs 43.7% in NHP (p=0.008). 3y DFS was 62.4% in HP vs 57.1% in NHP (p=0.08). In HP when stratified by MRD flow negativity post induction, there was no statistically significant difference in 3y OS (79.1% vs 90%) (p=0.4), 3y DFS 65.9% vs 60.4% (p=0.8) and 3y EFS 65.9% vs 52.9% (p=0.66). In NHP when stratified by MRD flow negativity post induction, there was also no statistically significant difference in 3y OS (100% vs 88.9%) (p=0.56), 3y DFS (75% vs 67.5%) (p=0.84) and 3y EFS (75% vs 67.5%) (p=0.8).
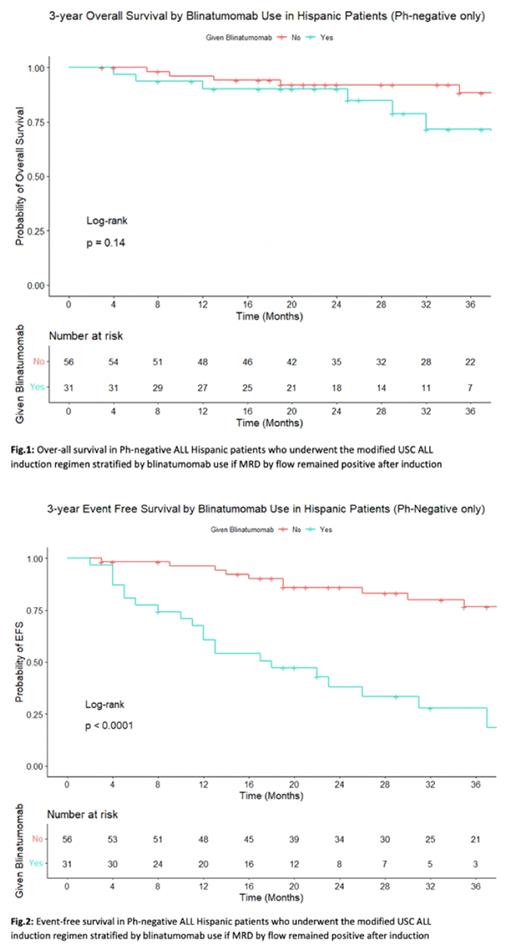
Of note, when survival is stratified by blinatumomab use, in HP: 3y OS 71.6% vs 88.3% (p=0.14), 3y EFS was 27.8% vs 76.6% (p=0.001), 3y DFS was 37.2% vs 76.6% (p=0.001) (Fig 1-2). In NHP: 3y OS 72.7% vs 77.7% (p=0.56), 3y EFS was 36.4% vs 44.4% (p=0.2), 3y DFS was 42.4% vs 64% (p=0.02).
No clear difference in grade 3/4 PEG toxicities were found in HP compared to NHP except grade 4 hypertriglyceridemia which was noted more in HP 31.3% compared to 9.7% in NHP (p=0.019).
CONCLUSIONS:
Our updated data shows a trend to better outcomes in HP compared to NHP in terms of OS and DFS, and a statistically significantly better EFS in the Hispanic population. Having MRD negativity by flow showed no statistically significant difference in both groups.
Of note, 3y EFS and 3y DFS were significantly lower in HP who received blinatumomab since these patients were MRD flow positive and lacked deeper remissions, but 3y OS (71.6%) remained high regardless, even though 26.4% who received blinatumomab were not able to go for allo-HSCT. In NHP, the trend is like that of HP but less pronounced. This suggests the beneficial effect of blinatumomab especially in Hispanic patients. Further follow-up is necessary to provide a more robust and mature survival data.
Disclosures
Chaudhary:Allogene: Current equity holder in publicly-traded company; TCR2: Current equity holder in publicly-traded company; Celldex: Current equity holder in publicly-traded company; Moderna: Current equity holder in publicly-traded company; Angeles Therapeutics: Consultancy, Current equity holder in private company; Pancella: Consultancy; Oncotartis: Consultancy; Athelas: Consultancy. Douer:Amgen: Speakers Bureau; Servier: Consultancy, Speakers Bureau; Adaptive Biotechnologies: Speakers Bureau. Yaghmour:USC: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.